Luisa®
Life Supporting Ventilation with High Flow Oxygen Therapy

Partnership for:
Better Patient Care
With over 60 years combined experience in respiratory care, Movair and Löwenstein Medical have partnered to deliver high-quality ventilation to the United States. The LUISA non-invasive/invasive ventilator with High Flow Therapy is a 3rd generation life supporting ventilator that represents the highest standards in German and U.S. product development, engineering, and manufacturing.

Movair, formerly International Biophysics Corporation, innovator of the AffloVest, has been in the cardiac surgery and respiratory market for over 30 years. Based in Austin, Texas, Movair advances life-empowering respiratory technologies that help people breath better and live better.

Löwenstein Medical is a global market leader in respiratory care. They have been providing innovative solutions that improve the care of patients with over 30 years designing and manufacturing high performance ventilators used by patients ranging from neonatal to the elderly in over 130 countries worldwide.
Understanding
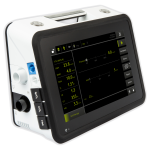
Luisa® at a Glance
The full featured LUISA from Movair and Löwenstein Medical is an advanced, easy-to-use, portable and compact home ventilator. LUISA has been globally recognized for multiple design excellence awards for intuitive, simplified everyday use.1
- TTV-VAPS-AE with unique Comfort Settings
- Sensitive Inspiratory and Expiratory Triggers with Inspiratory Lockout
- Three Target Speeds
- Pressure Increase and Pressure Reduction
- Flow-Based Auto-EPAP
- Auto-Rate algorithm
- High Flow Oxygen Therapy in all circuit systems
- No adapters needed
- 10” angled touchscreen
- Ease of Use programming
- 2nd alarm language
- Lightweight at ~ 8 lbs.
- Sp02 and Fi02 monitoring capabilities
- Up to 18 hours battery life2
- Customized patient downloadable reporting
Movair is the exclusive sales, clinical and service partner for LUISA in the U.S.A

High flow oxygen therapy
For Your Ventilator Patients
The LUISA, with High Flow Therapy (HFT), is a home ventilator that offers all the potential benefits of HFT for patients that meet the clinical requirements for home ventilation. High Flow Therapy can deliver a high flow blend of air and oxygen, through a high-flow nasal cannula, which can improve oxygenation and decrease the workload of breathing in a wide array of patient populations.3
High Flow Therapy has been used in critical care, emergency departments, and is now available to the ventilator patient at home.
High Flow Therapy provides a flow rate that meets or exceeds a patient’s inspiratory flow demand. This high flow rate can reduce the work of breathing and more closely match inspiratory demand through adjustable oxygen delivery and flow dependent clearance of carbon dioxide.4,5
High Flow Therapy that's
Easy to Use
LUISA has the potential to help manage nocturnally ventilated patients during the day with a less obtrusive oxygen flow delivery method that can potentially lead to:
Enhanced oxygenation compared to conventional low-flow oxygenation4
Improved work of breathing5
Better secretion clearance5
Increase in activities of daily living like eating, drinking and talking5
Superior comfort and less anxiety or claustrophobia which could lead to better compliance6
The LUISA provides the flexibility of High Flow Oxygen Therapy, for your patients that meet clinical requirements for home ventilation, in all circuits with a flow range of 5-60 lpm.
LUISA offers all the benefits of High Flow Therapy for your ventilator patients in one device at home.
Tailored Therapy
LUISA provides ventilator support and utilizes all standard volume, pressure and mouthpiece ventilation modes with the added benefit of High Flow Therapy. Ventilator modes include LUISA’s targeted tidal volume, volume-assured pressure support, auto EPAP (TTV-VAPS-AE).
Portability
Internal and external batteries offer long-lasting battery life with up to 18 hours of use. LUISA is a lightweight and portable ventilator that offers two position functionality for flexibility of use at bedside, in an easy-to-carry durable bag or mounted on a wheelchair.
Ease of Use
The LUISA is designed for simple set-up and operation. A large, angled and rotatable 10-inch touchscreen provides an easy to view screen when programming the device through its’ intuitive menu. It also provides easy visibility for patients from many angles while in use. The LUISA is a multi-purpose ventilator that does not require any adapters for different circuits.
Personalized reports
Device connectivity allows for personalized therapy data downloads. The customized reports provide essential information to manage your respiratory patients and ensure they are maximizing their therapy.
We make it easy
Service Simplified

LUISA with High Flow Therapy provides long term care with easy serviceability. Routine patient maintenance consisting of only filter changes and a device wipe down provides uncomplicated upkeep.
With simple, routine maintenance and no adapters required between circuits, the LUISA provides clinicians and patients peace of mind with an all-encompassing system in a compact, home ventilator with the added benefit of High Flow Therapy.
Take a look at our
Luisa Overview
High Flow Therapy
all circuit systems
Connectivity
customized patient
download reports
Uncomplicated
no adapters, intuitive
operation
Battery run time
up to 18 hours2
Monitoring
Fi02 and Sp02
measurement
Safety
alarm displayed in
two languages
Display
10” angled touchscreen
Functionality
100 – 3,000 mL Vt without
additional flow sensor
Responsive adjustable target
volume speed
Lightweight ~ 8 lbs.
Positioning vertical or horizontal;
Circuit orientation options (left or right side)
The LUISA has been authorized by the FDA under an EUA but has not been FDA cleared or approved. The LUISA is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner. U.S. federal law restricts this device to sale by or on the order of a physician.
1. iF Design Award for Design Excellence 2021 and Red Dot Award for Product Design 2021
2. Utilizing 6 hour internal battery plus two optional external batteries at 6 hours each.
3. Dysart, K. et al. Research in High Flow Therapy: Mechanisms of Action. Respiratory Medicine. 2009. 103(10):1400-5
4. Lodeserto, F. et al. High-Flow Nasal Cannula: Mechanisms of Action and Adult and Pediatric Indications. Cureus. 10(11):e3639.
5. Drake, M. High-Flow Nasal Cannula Oxygen in Adults: An Evidence Based Assessment. Ann Am Thorac Soc Vol.15(2):145-155.
6. Masclans, J. et al. The Role of High-Flow Oxygen Therapy in Acute Respiratory Failure. Medicina Intensiva. 2015; 39:505-515.


